Abstract
Background Tisagenlecleucel (Kymriah®), a CD19-directed chimeric antigen receptor T-cell (CAR-T) therapy, is standard of care (SOC) for patients (pts) with relapsed/refractory large B-cell lymphoma (r/r LBCL). Successful expansion and persistence of CAR-T cells strongly predicts response to this therapy. NT-I7 (efineptakin alfa) is a first in-class, long-acting human IL-7 that increases the number and functionality of T-cells in peripheral blood and within tumors. We hypothesize that NT-I7 administration after tisagenlecleucel SOC for pts with r/r LBCL may increase expansion and persistence of CAR-T, increasing tumor response rate and improving clinical outcomes without safety concerns.
Methods This phase 1b study consists of a dose-escalation phase followed by a dose expansion. In dose escalation, subjects receive tisagenlecleucel infusion on Day 0 and a single intramuscular dose of NT-I7 on Day 21, at 7 dose levels (DL1-7): 60, 120, 240, 360, 480, 600, and 720 μg/kg. DL1-2 enrolled 1 subject each, with the remaining DLs following a 3+3 design. Up to 42 subjects will be enrolled for the dose escalation phase. Eligible pts have biopsy-proven diagnosis of r/r LBCL after ≥2 lines of systemic therapy, are eligible for tisagenlecleucel as SOC, and have not yet received prior CD19-directed therapy. Primary objectives are to evaluate safety and tolerability and determine the maximum tolerated dose (MTD) and/or recommended phase 2 dose (RP2D) for NT-I7 with this regimen. Secondary objectives are to explore anti-tumor activity and the effect of NT-I7 after tisagenlecleucel on Grade ≥3 Cytokine Release Syndrome (CRS) and Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS). The effects of this regimen on expansion of absolute lymphocyte counts (ALC) and expansion of CAR-T cells will also be explored.
Results As of 30 May 2022, DL1-3 were closed and DL4 was still enrolling, with 7 pts enrolled total. Mean age was 67.1 years, with Eastern Cooperative Oncology Group (ECOG) status of 0 (2/7) or 1 (5/7). Lymphoma stage at diagnosis was Stage III-IV in 100% (7/7) of pts. At diagnosis, 57% (4/7) exhibited extranodal involvement without bulky nodal disease. None of the pts had double or triple hit phenotype. All pts had received ≥2 prior therapies, with 29% (2/7) post-autologous stem cell transplant.
All pts in DL1-3 (n = 5) completed the dose-limiting toxicity (DLT) period. No serious adverse events were observed. All pts experienced treatment-emergent adverse events (TEAEs), most of which were mild. 60% pts (3/5) experienced NT-I7-related AEs. 2 pts experienced Gr 1 injection site reaction. The other 2 NT-I7-related AEs, Gr 2 (1/3) and Gr 1 vomiting (1/3), were also considered immune-related AEs and AEs of special interest. Importantly, neither CRS nor ICANS were observed following NT-I7 treatment; and NT-I7 administration did not increase CRS-related inflammatory cytokines such as IL-6, IL-1beta, TNF, or IL-8.
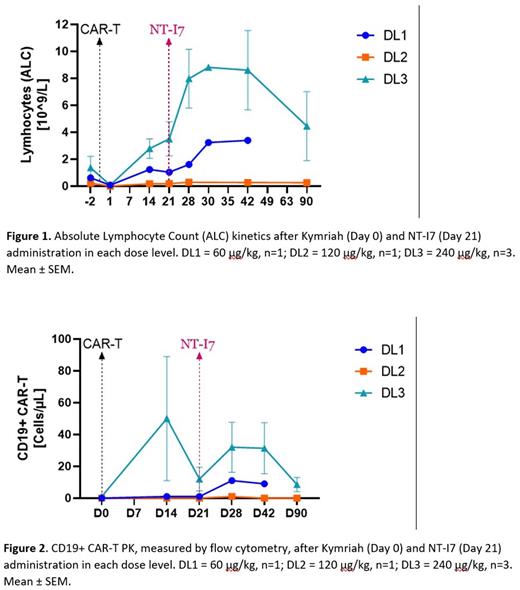
In DL1-3 pts, a single dose of NT-I7 at day 21 post-tisagenlecleucel significantly increased peripheral ALC, an effect that was most pronounced at DL3 (Figure 1). Meaningfully, a single dose of NT-I7 at the CAR-T contraction phase (day 21) was able to increase the CAR-T absolute numbers, measured by flow cytometry (Figure 2). Despite the limited number of patients enrolled in the lowest NT-I7 dose levels, and CAR-T levels being near the limit of assay detection, this increase is promising and may have strong clinical implications. Regarding preliminary antitumor activity in these pts, the best overall response as determined by the investigator was progressive disease (PD) in cohort 1 (day 30) and partial response in cohort 2, progressing at day 90. All pts at DL3 achieved BOR of complete response (CR, 2/3 and CMR, 1/3) at day 30, with 1 progression at day 90.
Conclusions In summary, NT-I7 treatment following tisagenlecleucel SOC was safe and well-tolerated. NT-I7 did not induce CRS or ICANS, nor proinflammatory cytokines. Notably, NT-I7 treatment led to a sustained increase in ALC and increased CAR-T cell absolute numbers in the peripheral blood. Preliminary antitumor efficacy was also observed, particularly at DL3. We conclude that the encouraging safety profile and early efficacy results warrant further investigation of NT-I7 in combination with tisagenlecleucel.
Disclosures
Ghobadi:Celgene: Consultancy; Wugen Inc: Consultancy; Atara: Consultancy; Amgen: Consultancy, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding. Budde:KITE: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Merck: Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Mustang Therapeutics: Research Funding; AstraZeneca: Research Funding; Amgen: Research Funding; Amgen: Research Funding. Galal:Kite Pharma: Consultancy, Speakers Bureau; Incyte: Speakers Bureau; Beigene: Speakers Bureau; EUSA: Consultancy, Speakers Bureau. Stermer:NeoImmuneTech, Inc.: Current Employment. Bierly:NeoImmuneTech, Inc.: Current Employment. Ferrando-Martinez:NeoImmuneTech, Inc.: Current Employment. Lee:NeoImmuneTech, Inc.: Current Employment, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company. DiPersio:Amphivena Therapeutics: Research Funding; NeoImmune Tech: Research Funding; Macrogenics: Research Funding; BioLineRx, Ltd.: Research Funding; CAR-T cell Product with Washington University and WUGEN: Patents & Royalties; VLA-4 Inhibitor with Washington University and Magenta Therapeutics: Patents & Royalties; hC Bioscience, Inc.: Membership on an entity's Board of Directors or advisory committees; RiverVest Venture Partners: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Research Funding; WUGEN: Current equity holder in private company, Research Funding; Magenta Therapeutics: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal